From Cell to Stack to System
Flow batteries separate the storage of electrical energy from the charge/discharge process; power and energy can be scaled independently of each other to meet the needs of many different applications. Very few technologies are realistically applicable to utility-scale electrical energy storage (EES); flow batteries are in an elite category. But they are also scalable to home and building applications for peak shaving or peak shifting, or for emergency backup power.
In a flow battery, energy storage is accomplished in arbitrarily large tanks full of electrolytes, while separate stacks of cells convert electricity into and out of different electrochemical (redox) states of those electrolytes. The tank on the left contains the catholyte, and the tank on the right contains the anolyte.

By Nick B, benboy00 - https://commons.wikimedia.org/wiki/File%3ARedox_Flow_Zelle_Deutsch_Farbverlauf.png, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=35143999
Of course, the single cell in the above diagram is actually a stack of about 14-19 cells in a real battery, a few of which are shown expanded below with both the membrane (typically DuPont NafionTM 117) and the porous graphite fiber electrode pads visible.
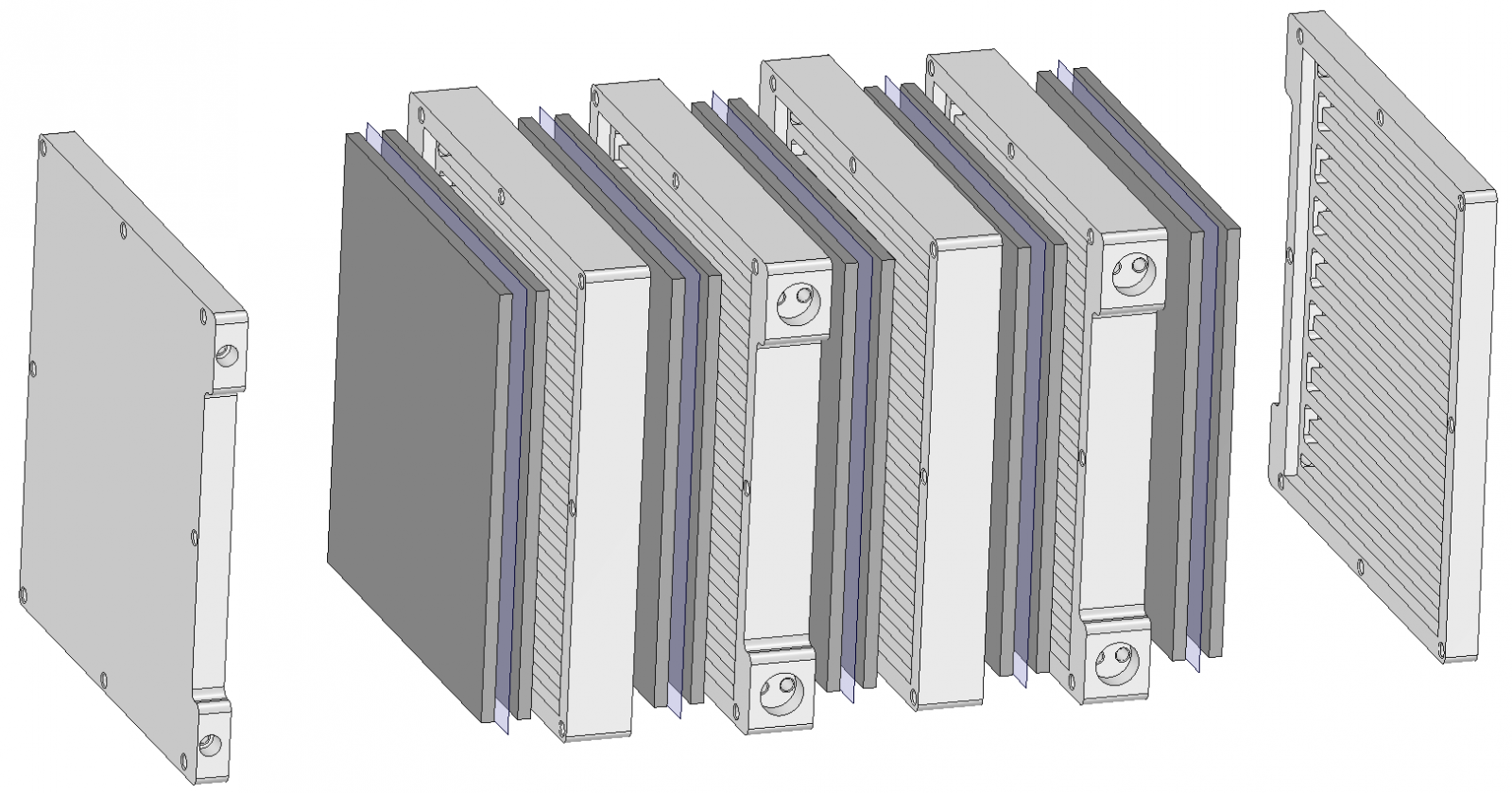
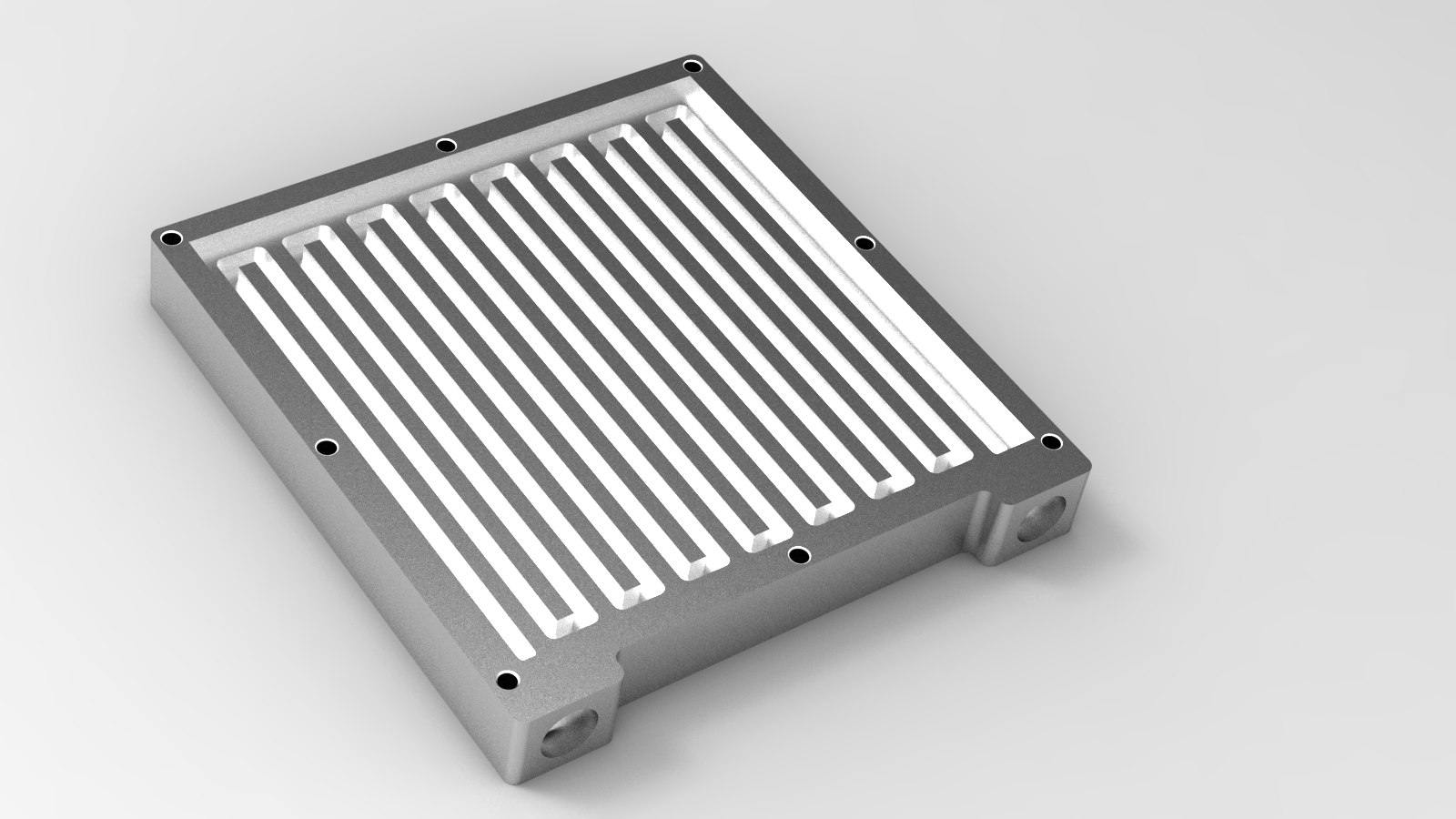
The charge collector plates also contain a serpertine "flow field" in the picture below, which is used to assure uniform distribution of electrolyte within the cell for minimum overpotential and reduced pumping losses.
There are many such plates in a stack, and many stacks are then plumbed in parallel in a battery. Time scales within a cell are on the order of fractions of a second, but any simulation of a flow battery must cover many days of charge/discharge scenarios.
This means that the complexities of modeling a realistic flow battery involve more than just combining fluid flow, thermal energy, electrical networks, and electrochemical treatment of redox reactions. They must also involve handling multiple time and distance scales simultaneously: you need to be able to zoom in on the fast time-scale multiphysics within a cell, while at the same time zooming out to the full system as it moves through its daily operational cycle.

Vanadium Redox Flow Batteries (VRFB) represent the current state-of-the-art, with 20+ years of reliable and safe operation demonstrated. Many research projects are underway to find alternate electrochemistries or membranes, or to reduce the cost and increase the performance of VRFBs.
Or just enjoy the diurnal variations of temperature gradients within a single cell of a 17-cell stack in a 6-stack battery over the course of a typical day:
Click here to fetch the VRFB case study on our User Forum
